- Stock: In Stock
- Model: 182280
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
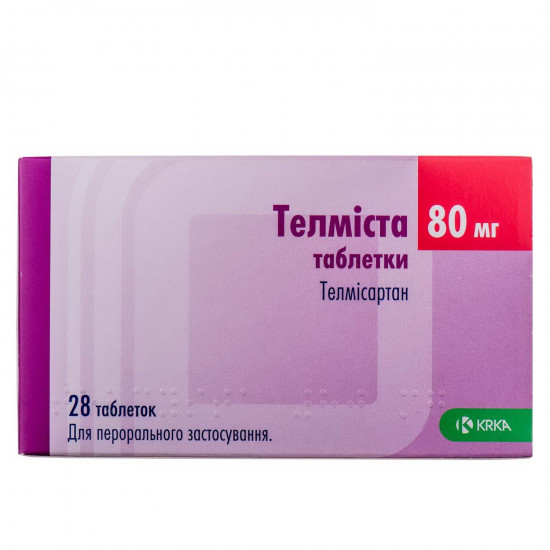
Reviews Over Telmista of the tab. of 80 mg No. 28
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
tablets "Telmista" are applied at the following indications:
- hypertensia: treatment of essential hypertensia at adults;
- prevention of cardiovascular diseases: decrease in incidence on cardiovascular diseases at patients with:
- to manifest aterotrombotichesky cardiovascular diseases (coronary heart disease, stroke or damages of peripheral arteries in the anamnesis);
- diabetes of the II type with the documented damage of target organs.
Structure
Active ingredient - telmisartan (one tablet contains 80 mg of a telmizartan).
Excipients: povidone, Megluminum, sodium hydroxide, lactoses monohydrate, sorbite (E 420), magnesium stearate.
Contraindication
- hypersensitivity to medicament components;
- pregnant women or women who planiruyu to become pregnant;
- obstructive biliary disturbances;
- heavy abnormal liver functions; simultaneous use of a telmizartan and aliskiren to patients with diabetes or renal failures (SKF <60 ml/minute / 1.73 to m 2 ) it is contraindicated to
- .
Route of administration
Drug "Telmista" is taken 1 time a day orally with enough liquid, together with food or without it.
Treatment of arterial hypertension
Usual effective dose makes 40 mg a day. To some patients the daily dose of 20 mg can be sufficient. In case desirable arterial blood pressure is not reached, the dose of a telmizartan can be raised to 80 mg of 1 times a day. Alternatively telmizartan it is possible to appoint in a combination with thiazide diuretics, such as hydrochlorothiazide which showed an additional lowering of arterial pressure at use with telmisartany. When the question of increase in a dose is considered, it is necessary to take into account that the maximum antihypertensive effect in general is reached in 4-8 weeks from an initiation of treatment.
Prevention of cardiovascular diseases
Recommended dose makes 80 mg of 1 times a day. The efficiency of a telmizartan in doses less than 80 mg at prevention of cardiovascular diseases is unknown.
Beginning withtreatment telmisartany for the purpose of prevention of cardiovascular diseases, it is recommended to carry out monitoring of arterial blood pressure and in case of need - to adjust a dose of the medicaments reducing arterial blood pressure.
ChildrenDrug "Telmista" is not recommended to
Feature of use
by for use for children aged up to 18 years because of insufficiency of data on safety and efficiency. Drivers
When driving and mechanisms needs to consider possibility of dizziness or a hypersomnia at antihypertensive therapy, including reparaty Telmista.
toOverdose
Is available toonly limited information on overdose at the person.
Symptoms
Most considerable manifestations of overdose of a telmizartan were arterial hypotension and tachycardia; also there were cases of bradycardia, dizziness, increase in creatinine in blood serum and an acute renal failure.
Treatment
Telmizartan does not leave by a hemodialysis. In case of overdose for the patient it is necessary to establish careful observation and to appoint symptomatic and maintenance therapy. The choice of treatment depends on time which passed after introduction, and from weight of symptoms. It is necessary to consider such measures as stimulation of vomiting and/or gastric lavage. Activated carbon can be useful in overdose treatment. It is necessary to control often the level of electrolytes and creatinine in blood serum. At appearance of arterial hypotension of the patient it is necessary to put in a prone position and to quickly enter substitutes of salt and to restore liquid volume.
Side effectsSerious by-effects including anaphylactic reactions and a Quincke's disease are possible
in rare instances (from ≥ 1/10000 to <1/1000) and also the acute renal failure was observed. Storage conditions
to Store
in original packing for protection against effect of light at a temperature not above 30 °C, out of children's reach.
Expiration date - 3 years.
Specifications
| Characteristics | |
| Active ingredients | Telmisartan |
| Amount of active ingredient | 80 mg |
| Applicant | KRK |
| Code of automatic telephone exchange | C09CA07 Telmizartan |
| Interaction with food | It doesn't matter |
| Light sensitivity | Sensitive |
| Market status | The branded generic |
| Origin | Chemical |
| Prescription status | According to the prescription |
| Primary packing | blister |
| Producer | KRK D.D. |
| Quantity in packing | 28 tablets (4 blisters on 7 pieces) |
| Release form | tablets for internal use |
| Route of administration | Oral |
| Sign | Import |
| Storage temperature | from 5 °C to 30 °C |
| Trade name | Telmista |




















