

- Stock: In Stock
- Model: 179718
0% Customers recommend this product
-
5 Awesome0%
-
4 Great0%
-
3 Average0%
-
2 Bad0%
-
1 Poor0%
Reviews Over Sandimmun Neoral kaps. myagk. 25 mg No. 50
- (0)
Total Reviews (0)
click here write review to add review for this product.
Report this review.
Description
Capsules soft Sandimmun Neoral are appointed at the following indications:
Indication at transplantations
- Transplantation of solid bodies:
- prevention of rejection of transplants of solid bodies;
- treatment of graft rejection at the patients who were earlier receiving treatment by other immunosuppressive medicines.
- Bone marrow transplantation:
- prevention of rejection of an allogenic transplant of marrow and transplant of stem cells;
- prevention and treatment of reaction "transplant against the owner".
Indication, not connected with transplantation
- Endogenous uveitis:
- active average or back uveitis menacing with loss of sight, a non-infectious etiology in cases when alternative treatment was inefficient or unacceptable because of side reactions;
- a uveitis at Bekhchet's disease with repeated aggravations of inflammation with involvement of a retina of an eye without neurologic symptomatology.
- Nephrotic syndrome:
- a steroidozavisimy or steroidorezistentny nephrotic syndrome owing to the minimum changes at primary glomerulonephritis, a focal segmentary glomerulosclerosis or a hymenoid glomerulonephritis.
- Induction or remission maintenance:
- maintenance of remission, caused by corticosteroids that does them to cancellation.
- Pseudorheumatism:
- treatment of severe forms of an active pseudorheumatism.
- Psoriasis:
- severe forms of psoriasis when standard treatment was inefficient or unacceptable.
- Atopic dermatitis:
- treatment of severe forms of atopic dermatitis in need of system therapy.
Structure
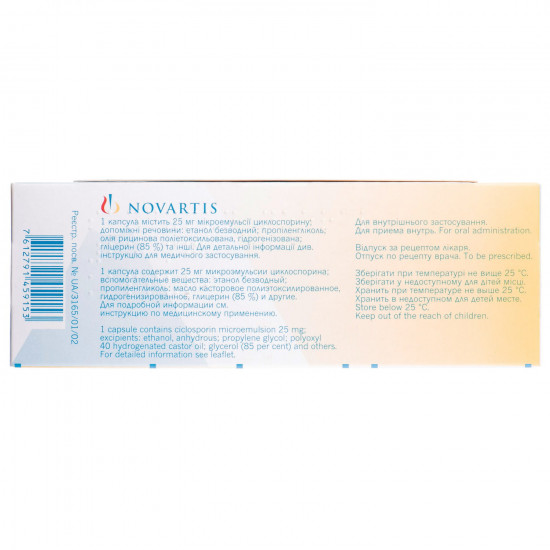
Active ingredient: ciclosporin;
1 capsule contains 10 or 25 or 50, or 100 mg of a microemulsion of cyclosporine;
Excipients: the castor oil of a poliyetoksilyovan hydrogenated mono - and di triglycerides of corn oil; ethanol; propylene glycol; α-tocopherol;
Cover of capsules: gelatin, propylene glycol, glycerin (85%), residual solvents, the titan dioxide (E 171), ferrous oxide black (E172) - only for capsules of 25 mg and 100 mg;
carminic acid (E 120), aluminum Blackened red food chloride hexahydrate, sodium hydroxide, propylene glycol, a gipromelloza, isopropyl alcohol, the water purified.
Contraindication
Hypersensitivity to cyclosporine or to any of medicine excipients. Simultaneous application with the medicines containing Hypericum perforatum (the St. John's wort which is made a hole) because of risk of decrease in concentration of cyclosporine in blood and, thus, decrease in therapeutic effect.
Simultaneous application with medicines, are substrates of the multilikarsky efluxny carrier of the R-glycoprotein (Pgp) or the organic anions of transport proteins (OATP) which increase in concentration in blood plasma is connected with development of serious side reactions and/or side reactions, life-threatening, for example with bozentany, a dabigatrana eteksilaty and aliskireny.
such contraindications are Also possible:
- Renal failure, except for patients with a nephrotic syndrome and moderately increased initial concentration of creatinine at most up to 200 µmol/l at adults and 140 µmol/l is at children. At a nephrotic syndrome the careful treatment using doses not higher than 2.5 mg/kg/days, only is allowed when use of cyclosporine contributes to normalization of the indicators of creatinine raised as a result of a disease.
- Insufficiently controlled hypertensia.
- Insufficiently controlled infection.
Existence in the anamnesis of the known or diagnosed malignant new growths of any kind, except for precancerous conditions or malignant damages of skin after treatment.
Route of administration
recommends to apply Sandimmun Neoral® according to the accurate schedule taking into account time of day and a diet. If the mode of dosing is registered it is impossible to provide by means of capsules, in particular for patients with low body weight, it is recommended to apply oral solution.
Sandimun of Neoral® can appointonly the doctor having experience of performing immunosuppressive therapy and/or organ transplantation.
Pregnant
not to apply
Feature of application
during pregnancy, except cases when the expected advantage for mother exceeds potential risk for a fruit. ChildrenUse to children aged up to 16 years on the indications which are not connected with transplantation, except a nephrotic syndrome, cannot be
recommended. Overdose
Symptoms. Data on sharp of overdose of cyclosporine are limited. Oral administration of doses up to 10 g (about 150 mg/kg) led to rather insignificant clinical consequences, such as vomiting, drowsiness, headache, tachycardia, and at some patients - to rather big reverse renal failures. However the accidental parenteral overdose at premature babies led to heavy intoxication.
Treatment. In all cases of overdose it is necessary to hold symptomatic treatment and the general supporting events. During the first hours after overdose can be useful to cause vomiting and to carry out gastric lavage. Drug is practically not removed at a hemodialysis and insufficiently removed at hemoperfusion using activated carbon.
Side effects
K to the main undesirable reactions, noted during clinical trials and connected with use of cyclosporine, belong renal failures, a tremor, a hirsutism, arterial hypertension, diarrhea, anorexia, nausea and vomiting.
Interaction
Oktreotid reduces oral absorption of cyclosporine owing to what increase in a dose of cyclosporine by 50% or transition to a dosage form for intravenous administration can be required.
byAt simultaneous application of grapefruits or grapefruit juice noted growth of bioavailability of cyclosporine.
Azithromycin increases concentration of cyclosporine approximately by 20%.
Storage conditionsto Store
at a temperature not above 25 °C. To store out of children's reach.
Expiration date - 2 years.
Specifications
| Characteristics | |
| Active ingredients | Cyclosporine |
| Amount of active ingredient | 25 mg |
| Applicant | Novartis |
| Code of automatic telephone exchange | L04AD01 Cyclosporine |
| Interaction with food | It doesn't matter |
| Light sensitivity | Not sensitive |
| Market status | Original |
| Origin | Chemical |
| Prescription status | According to the prescription |
| Primary packing | blister |
| Producer | NOVARTIS PHARM STEIN AG |
| Quantity in packing | 50 capsules (10 blisters on 5 pieces) |
| Release form | capsules for internal use |
| Route of administration | Oral |
| Sign | Import |
| Storage temperature | from 5 °C to 25 °C |
| Trade name | Sandimmun |




















